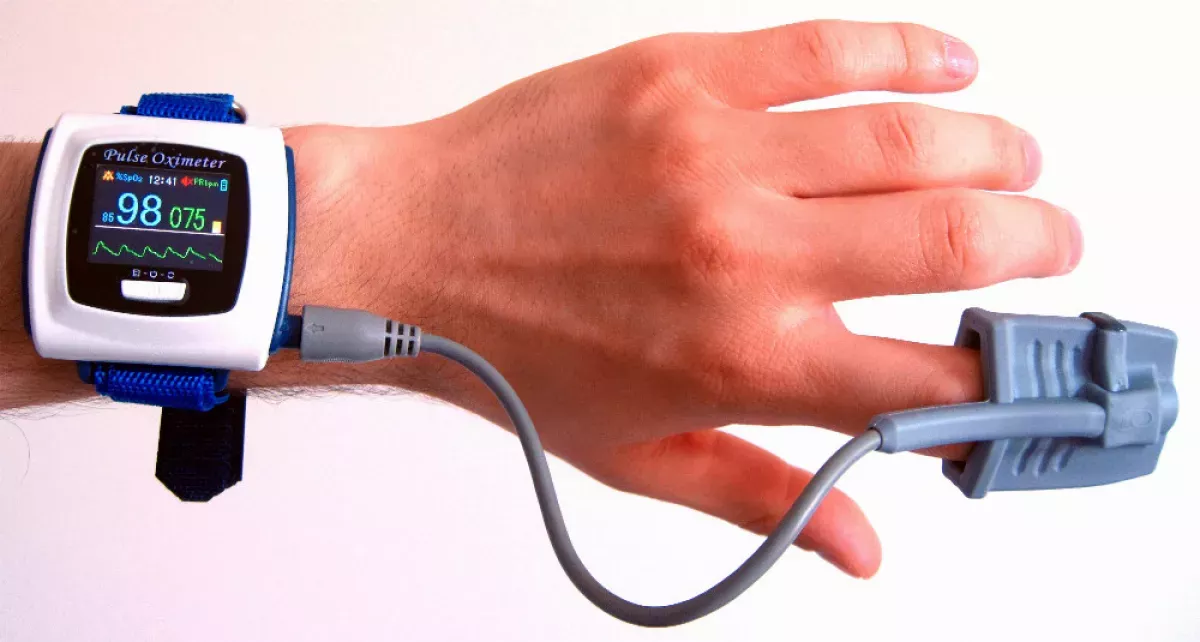
Pulse oximetry is a non-invasive technique used to measure blood oxygen levels. It determines the percentage of oxygen carried by red blood cells, providing a value known as peripheral oxygen saturation (SpO2). This method is widely recognized for its convenience and is generally accurate, with SpO2 readings typically falling within a 2% range of the more precise arterial oxygen saturation (SaO2) measurements obtained through arterial blood gas analysis.
1935: First Two-wavelength Ear O2 Saturation Meter
German physician Karl Matthes (1905–1962) pioneered the first two-wavelength ear O2 saturation meter in 1935. This device, utilizing red and green filters (later substituted with red and infrared filters), marked the inception of O2 saturation measurement technology.
1943: Modification of the Oximeter
In 1943, Earl Wood enhanced the oximeter by incorporating a pressure capsule to temporarily squeeze blood out of the ear. This method aimed to achieve an absolute O2 saturation reading when the blood flow returned.
1949: Publication of Wood's Oximeter Modification
Earl Wood's modified oximeter design, featuring a pressure capsule for absolute O2 saturation measurement, was published in 1949.
1964: First Absolute Reading Ear Oximeter
In 1964, Shaw spearheaded the creation of the first ear oximeter capable of providing absolute readings, utilizing eight distinct wavelengths of light.
1972: First Pulse Oximetry Development
Japanese bioengineers Takuo Aoyagi and Michio Kishi achieved a significant breakthrough in 1972 by developing the first pulse oximeter. This innovation, based on the ratio of red and infrared light absorption in pulsating blood, marked a pivotal step in noninvasive blood oxygen monitoring.
1975: First Pulse Oximeter Testing on Patients
Following its development, the first pulse oximeter underwent patient testing in 1975, conducted by surgeon Susumu Nakajima and his colleagues. This marked the first instance of this technology's use in a clinical setting.
1976: Early Study on Pulse Oximetry Inaccuracies in Dark-Skinned Patients
A 1976 study first identified that pulse oximeter devices may produce inaccurate readings for patients with darker skin tones. The study revealed that these devices often showed lower blood oxygen saturation values than were actually present in these patients.
1977: First Commercial Finger Pulse Oximeter
In 1977, Minolta introduced the OXIMET MET-1471, marking the first commercialization of a finger pulse oximeter. This development made pulse oximetry technology more accessible for practical use.
1980: First Pulse Oximeter Commercialization in the U.S.
Biox introduced the first commercially available pulse oximeter in the United States in 1980, marking a significant milestone in the technology's widespread adoption within the country's healthcare system.
1987: Pulse Oximetry as Standard of Care
By 1987, the use of pulse oximetry became a standard procedure in the United States during the administration of general anesthesia.
1995: Introduction of Signal Extraction Technology (SET) and Perfusion Index
In 1995, Masimo introduced Signal Extraction Technology (SET) to improve pulse oximetry accuracy during patient motion and low perfusion. This technology differentiates arterial signals from venous and other signals, addressing limitations of existing methods that relied on averaging times or freezing values. The same year, Masimo also introduced the perfusion index, which quantifies the amplitude of the peripheral plethysmograph waveform, aiding clinicians in predicting illness severity and potential complications.
2005: Pulse CO-oximeter
In 2005, Masimo developed the pulse CO-oximeter, a noninvasive method for continuous dyshemoglobin measurement. This technology utilizes additional wavelengths to measure carboxyhemoglobin, methemoglobin, and total hemoglobin alongside dyshemoglobins.
2007: Introduction of Pleth Variability Index (PVI)
In 2007, Masimo pioneered the measurement of the Pleth Variability Index (PVI). This noninvasive method offered a novel approach to assessing a patient's response to fluid administration, a crucial factor in postoperative care.
2010: Landmark Study on Signal Extraction Technology Impact
A landmark study published in 2010 demonstrated the significant impact of Signal Extraction Technology (SET) on patient care. The study, conducted at Dartmouth-Hitchcock Medical Center, revealed that using SET pulse oximetry on the general floor led to a decrease in rapid response team activations, ICU transfers, and ICU days.
2011: Recommendation for Newborn Screening with Pulse Oximetry
In 2011, an expert workgroup recommended incorporating pulse oximetry into newborn screening procedures. The goal was to improve the detection of critical congenital heart disease (CCHD). The workgroup specifically highlighted the efficacy of motion-tolerant and low-perfusion validated pulse oximetry, citing results from large-scale studies. In the same year, the US Secretary of Health and Human Services added pulse oximetry to the recommended uniform screening panel, leading to a significant increase in newborn screening rates.
2011: US Pulse Oximetry Market Value
In 2011, the US market for pulse oximetry monitoring equipment and sensors surpassed $700 million, as reported by iData Research.
2014: Confirmation of Pulse Oximetry Effectiveness in Newborn Screening
Further reinforcing the importance of pulse oximetry in newborn screening, a third large-scale study in 2014 affirmed the positive results observed in previous studies. The study, involving 122,738 newborns, solidified the effectiveness of this technology in detecting critical congenital heart disease.
2020: Follow-up Study on Long-Term Benefits of Signal Extraction Technology
In 2020, a follow-up study at Dartmouth-Hitchcock Medical Center reinforced the long-term benefits of Signal Extraction Technology. This retrospective study, spanning ten years of using SET pulse oximetry alongside a patient surveillance system, reported zero patient deaths and no instances of patient harm from opioid-induced respiratory depression while continuous monitoring was employed.
Mentioned in this timeline
The heart a muscular organ in humans and animals pumps...
Trending

1 month ago Bradley Cooper directs Will Arnett and Laura Dern in the dramedy 'Is This Thing On?'

Steve Nash is a retired Canadian professional basketball player and former head coach of the Brooklyn Nets Renowned for his...

3 months ago Kevin Bacon's 'Tremors' revival, 'The Bondsman' series, and 90s monster movie acclaim.

9 months ago Billy Bob Thornton's 'Landman' Season 2 Starts Production; Demi Moore Returns.

James Jimmy G Garoppolo is a professional football quarterback currently playing for the Los Angeles Rams in the NFL He...

8 months ago George Kittle Discussing 49ers Extension Amidst Brock Purdy Contract Updates and NFL News
Popular

Stranger Things created by the Duffer Brothers is a popular...

XXXTentacion born Jahseh Dwayne Ricardo Onfroy was a controversial yet...
The Kennedy Center Honors are annual awards recognizing individuals and...
Turning Point USA TPUSA is an American nonprofit organization founded...

Candace Owens is an American conservative political commentator and author...

It's a Wonderful Life directed by Frank Capra tells the...