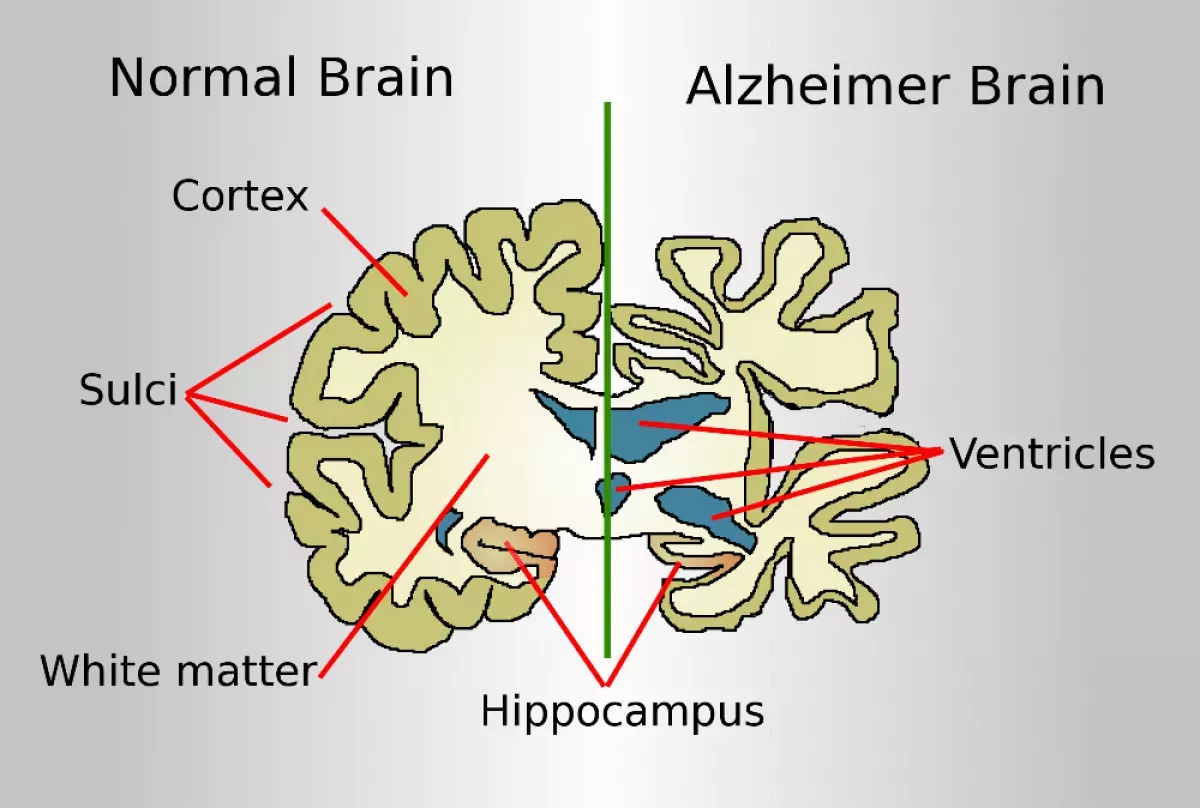
Alzheimer's disease (AD) is a neurodegenerative disease responsible for 60-70% of dementia cases. It progressively worsens, starting with difficulty remembering recent events. Advanced symptoms include language problems, disorientation, mood swings, loss of motivation, self-neglect, and behavioral issues, leading to social withdrawal. Bodily functions gradually decline, ultimately causing death. While the progression rate varies, the typical life expectancy after diagnosis is three to twelve years.
1901: First Case of Alzheimer's Disease Identified
Alois Alzheimer identified the first case of Alzheimer's disease in 1901, in a 50-year-old woman named Auguste D.
1906: First Public Report on Alzheimer's Disease
Following Auguste D.'s death in 1906, Alois Alzheimer publicly reported on her case, marking the first time the disease was formally documented.
July 1910: Alzheimer's Disease Recognized as a Distinct Disease
In July 1910, Emil Kraepelin officially distinguished Alzheimer's disease as a subtype of senile dementia, solidifying its recognition as a separate entity.
1977: Shift in Alzheimer's Disease Diagnosis
In 1977, a conference on Alzheimer's disease led to a change in how the disease was diagnosed. Experts concluded that the clinical and pathological signs of presenile and senile dementia were nearly identical, paving the way for age-independent diagnosis of Alzheimer's disease.
1984: NINCDS-ADRDA Alzheimer's Criteria Established
In 1984, the NINCDS and ADRDA established standardized criteria for diagnosing Alzheimer's disease, which relied on cognitive impairment and neuropsychological testing.
1991: Amyloid Hypothesis
The amyloid hypothesis, proposed in 1991, suggests that extracellular amyloid beta (Aβ) deposits are the primary cause of Alzheimer's disease. This is supported by the location of the amyloid precursor protein (APP) gene on chromosome 21 and the fact that individuals with Down syndrome (trisomy 21) often develop early Alzheimer's symptoms by age 40.
1995: William Utermohlen Begins Self-Portraits
Artist William Utermohlen began creating self-portraits in 1995 to document his experience with Alzheimer's disease.
1996: The Notebook Novel Publication
Nicholas Sparks's novel "The Notebook" was published in 1996.
1999: Malcolm and Barbara: A Love Story Documentary Release
The documentary "Malcolm and Barbara: A Love Story" was released in 1999.
2000: William Utermohlen Concludes Self-Portraits
William Utermohlen finished his series of self-portraits depicting his disease progression in 2000.
2001: Iris Film Release
The film "Iris," based on John Bayley's memoir about his wife Iris Murdoch, was released in 2001.
2004: The Notebook and A Moment to Remember Film Releases
Two films related to Alzheimer's, "The Notebook" and "A Moment to Remember," were released in 2004.
2005: Thanmathra Film Release
The film "Thanmathra" was released in 2005.
2006: Memories of Tomorrow and Away from Her Film Releases
"Memories of Tomorrow" and "Away from Her," both dealing with Alzheimer's themes, were released in 2006.
2007: Still Alice Novel Publication
Lisa Genova's novel "Still Alice" was published in 2007.
2007: NINCDS-ADRDA Criteria Updated
The NINCDS-ADRDA Alzheimer's Criteria were extensively updated in 2007.
2008: Osaka Mutation Discovery
In 2008, a deletion mutation of codon 693 of the APP gene, known as the Osaka mutation, was linked to familial Alzheimer's disease in a Japanese pedigree.
2008: Persistent Repetition of Phrases Music Release
The Caretaker released the album "Persistent Repetition of Phrases" in 2008.
2010: Risk of Dying from AD in the United States
In 2010, disparities in the risk of dying from Alzheimer's disease (AD) were observed among different racial and ethnic groups in the United States. Non-Hispanic white individuals faced a 26% higher risk compared to non-Hispanic Black individuals, while the Hispanic population had a 30% lower risk than the non-Hispanic white population.
2010: International Working Group Criteria for Alzheimer's Disease
The International Working Group updated its criteria for the diagnosis of Alzheimer's disease in 2010.
2011: An Empty Bliss Beyond This World Music Release
The Caretaker released "An Empty Bliss Beyond This World" in 2011.
2011: NIA-AA Definition of Alzheimer's Disease
The National Institute on Aging-Alzheimer's Association (NIA-AA) revised its definition of Alzheimer's disease in 2011.
2012: FDA Approval of Florbetapir
The FDA approved florbetapir as a radiopharmaceutical diagnostic agent for Alzheimer's disease in 2012.
2013: FDA Approval of Flutemetamol
Flutemetamol received FDA approval as a radiopharmaceutical diagnostic agent for Alzheimer's disease in 2013.
2013: DSM-5 Criteria for Alzheimer's Diagnosis
The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), published in 2013, provided a set of criteria for diagnosing Alzheimer's disease.
2014: FDA Approval of Florbetaben
In 2014, florbetaben was approved by the FDA as a radiopharmaceutical diagnostic agent for use in Alzheimer's disease.
2014: Still Alice Film Release
The film "Still Alice," based on Lisa Genova's novel, portrayed a Columbia University professor with early-onset Alzheimer's disease and was released in 2014.
2016: Review of Ginkgo biloba for Alzheimer's Treatment
A 2016 review concluded that there wasn't sufficient evidence to support using Ginkgo biloba for Alzheimer's treatment.
2016: Everywhere at the End of Time Music Release Begins
The Caretaker began releasing "Everywhere at the End of Time" in 2016.
2018: Review of Reminiscence Therapy for Alzheimer's
A 2018 review examining the effectiveness of reminiscence therapy (RT) for Alzheimer's disease found inconsistent and small effects with questionable clinical significance, varying depending on the setting.
2018: Limited Use of Advanced Imaging
Due to lack of insurance coverage, the use of advanced imaging techniques for Alzheimer's disease diagnosis remained mostly confined to clinical trials as of 2018.
2018: Celiac Disease and Dementia Link
In 2018, a review suggested a connection between celiac disease and several dementia types, including Alzheimer's.
2019: Celiac Disease and Dementia
A 2019 study found no overall increased risk of dementia, including Alzheimer's, in people with celiac disease.
2019: Cost of Informal Care for AD in the United States
Informal care for individuals with AD in the United States was estimated to cost $234 billion and 18.5 billion hours of care in 2019.
2019: Everywhere at the End of Time Music Release Concludes
The final installment of The Caretaker's "Everywhere at the End of Time" was released in 2019.
2020: Sleep Disturbances and Alzheimer's Disease
As of 2020, accumulating evidence indicated a potential bidirectional link between sleep disruption and Alzheimer's disease, suggesting that sleep problems may be both a cause and a consequence of the disease.
2020: Genetic Models for Alzheimer's Prediction
As of 2020, genetic models could predict Alzheimer's disease with 90% accuracy. Late-onset Alzheimer's was determined to be about 70% heritable.
2020: Global Alzheimer's Statistics
In 2020, around 50 million people globally had Alzheimer's disease. It primarily affects people over 65, though up to 10% of cases are early-onset, occurring in those aged 30 to mid-60s. It impacts about 6% of people aged 65 and older, with women more frequently affected than men.
2020: EU Dementia Research Funding
In 2020, the European Union's Horizon Europe research program granted over €570 million to projects related to dementia.
2020: Prevalence of AD in the United States
In 2020, the prevalence of AD dementia in the United States varied across age groups: 5.3% for ages 60-74, 13.8% for 75-84, and 34.6% for those over 85.
2020: FDA Approval of Flortaucipir
The FDA approved flortaucipir, another radiopharmaceutical diagnostic agent for Alzheimer's disease, in 2020.
2021: Aducanumab FDA Approval
Aducanumab received accelerated approval from the FDA for Alzheimer's treatment in 2021, but its effectiveness was questioned, particularly in individuals with severe symptoms, and Biogen later discontinued it in 2024.
July 2023: Lecanemab FDA Approval
Lecanemab received traditional FDA approval for Alzheimer's treatment in July 2023, following accelerated approval, but received a boxed warning for amyloid-related imaging abnormalities.
July 2024: Donanemab and Benzgalantamine FDA Approval
Both Donanemab, a plaque-clearing drug, and benzgalantamine, a cholinesterase inhibitor, were approved by the FDA in July 2024. It's important to note that anti-amyloid drugs like Donanemab can cause brain shrinkage.
2024: Aducanumab Discontinuation
Biogen discontinued aducanumab in early 2024.
2024: Focused Ultrasound for Drug Delivery Across the Blood-Brain Barrier
In 2024, a new method using focused ultrasound is being explored to enhance drug delivery across the blood-brain barrier (BBB). This non-invasive technique, which avoids incisions or radiation, concentrates ultrasound beams to create therapeutic effects at the convergence point, including opening the BBB. This opening could potentially facilitate the removal of substances like beta-amyloid and tau, which are implicated in Alzheimer's disease.
2026: US Alzheimer's Research Budget
The US National Institutes of Health allocated a budget of US$3.98 billion for Alzheimer's research in the fiscal year 2026 as part of the National Plan to Address Alzheimer's Disease.
2050: Projected Global Prevalence of AD
The global prevalence of Alzheimer's disease is projected to triple by 2050, reaching 152 million, up from 50 million in 2020.
2050: Projected Global Cost of AD Care
The worldwide cost of AD care is projected to reach approximately $9.1 trillion by 2050.
Mentioned in this timeline

Music is a cultural universal involving the arrangement of sound...

Columbia University located in New York City is a private...
Trending

14 minutes ago Oklahoma Sooners Honor Buddy Hield and 2016 Final Four Team.
14 minutes ago Moldova's Veterinary Education to modernize through the EDUVET European Project for enhancement.

15 minutes ago Brendan Fraser as Eisenhower in 'Pressure': D-Day drama unfolds with weather worries.

15 minutes ago Cade Cunningham's 42 Points Leads Pistons Over Knicks; Brunson's Performance Notable.

15 minutes ago Usha Vance's diaper request sparks controversy during pregnancy: A novel approach?

1 hour ago Learner Tien at Delray Beach Open: Fritz ousted, Paul advances, Korda previewed.
Popular

Jesse Jackson is an American civil rights activist politician and...

Barack Obama the th U S President - was the...

Bernie Sanders is a prominent American politician currently serving as...

Ken Paxton is an American politician and lawyer serving as...

Michael Joseph Jackson the King of Pop was a highly...
Randall Adam Fine is an American politician a Republican who...