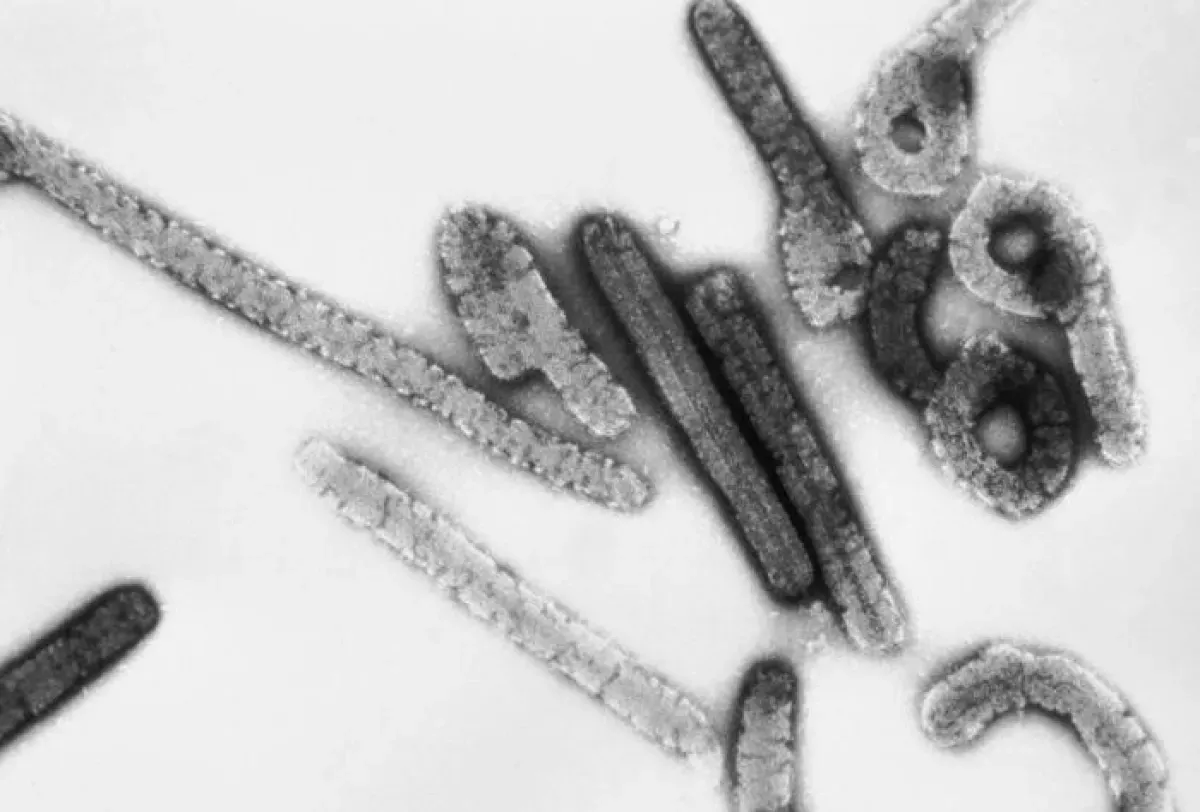
Marburg virus (MARV) is a severe hemorrhagic fever virus that affects primates. Classified as a high-risk pathogen by organizations like the WHO, NIAID, and CDC, it is considered a significant threat to public health and biosecurity. Its classification as a Category A bioterrorism agent highlights its potential for misuse and the importance of strict control measures.
1967: Marburg Virus Strains Isolated in Uganda
In 1967, Marburg virus strains were isolated in Uganda.
1967: Marburg Virus First Described
The Marburg virus was first described in 1967 following outbreaks of Marburg virus disease in Marburg and Frankfurt, Germany, and Belgrade, Yugoslavia. The outbreaks were linked to laboratory workers exposed to infected tissues from grivet monkeys at the Behringwerke facility in Marburg.
1967: Marburg Virus Named
The term "Marburg virus" was first introduced in 1967.
1999: Marburg Virus Strains Isolated in the Democratic Republic of the Congo
Marburg virus strains were isolated in the Democratic Republic of the Congo in 1999.
2000: Marburg Virus Strains Isolated in the Democratic Republic of the Congo
Marburg virus strains were isolated in the Democratic Republic of the Congo in 2000.
2004: Marburg Virus Strains Isolated in Angola
Marburg virus strains were isolated in Angola in 2004.
2005: Virus Name Changed to Lake Victoria marburgvirus
In 2005, the name of the virus was changed to Lake Victoria marburgvirus, differentiating the virus from its species only by italicization.
2005: Marburg Virus Strains Isolated in Angola
Marburg virus strains were isolated in Angola in 2005.
2007: Marburg Virus Strains Isolated in Uganda
Marburg virus strains were isolated in Uganda in 2007.
2009: Expanded Clinical Trials for Ebola and Marburg Vaccine Begin
In 2009, expanded clinical trials for a vaccine targeting both Ebola and Marburg viruses were initiated in Kampala, Uganda.
2009: Marburg Virus Strains Isolated in Uganda
Marburg virus strains were isolated in Uganda in 2009.
2009: Infectious MARV Isolated from Egyptian Fruit Bats
The successful isolation of infectious MARV from healthy Egyptian fruit bats in 2009 suggested a potential role for Old World fruit bats in the natural transmission cycle of marburgviruses. This finding, alongside the isolation of infectious RAVV, further supported this hypothesis.
2010: Marburg Virus Name Reinstated
The name "Marburg virus" was reinstated in 2010 due to the confusion caused by the 2005 name change. The species name was subsequently revised.
2012: Experimental Infection Study on Rousettus aegyptiacus
In 2012, an experimental infection study involving Rousettus aegyptiacus provided more evidence for the potential role of these bats in the ecology of MARV.
2014: Merck Acquires Rights to Candidate Vaccines
Merck acquired the rights to all of the related candidate vaccines in 2014, but they chose not to develop most of them, including the Marburg vaccine, due to financial considerations.
2014: First Clinical Trial for Marburg Virus Vaccine
The first clinical trial to assess the effectiveness of a Marburg virus vaccine took place in 2014. The trial focused on a DNA vaccine and found that vaccinated individuals developed some antibodies, although complete immunity was not anticipated.
2019: Merck Brings rVSV-ZEBOV Vaccine to Market
In 2019, Merck brought the rVSV-ZEBOV vaccine, which is closely related, into commercial use with funding from GAVI.
June 23, 2022: Study Shows Promising Results of rVSV Vaccine in Guinea Pigs
On June 23, 2022, researchers affiliated with the Public Health Agency of Canada released a study titled PHV01. The study showed promising results for a recombinant vesicular stomatitis virus (rVSV) vaccine tested on guinea pigs. It found that vaccinating guinea pigs approximately one month before infection provided a high level of protection.
Mentioned in this timeline
Germany officially the Federal Republic of Germany is a nation...
Kenya officially the Republic of Kenya is an East African...
Canada is a North American country the second largest in...
Ghana officially the Republic of Ghana is a West African...
Uganda officially the Republic of Uganda is a landlocked East...
Guinea officially known as the Republic of Guinea is a...
Trending

18 minutes ago Shaq Reveals Phil Jackson's Motivation Tactics; ESPN Changes 'Inside the NBA' Lineup.

18 minutes ago Jaden Ivey sidelined for weeks with knee soreness; Bulls confident on recovery.

18 minutes ago AJ Dybantsa: Potential $100 Million NBA Prospect, 2026 Mock Draft Predictions Emerge

19 minutes ago Kevin Durant praises Jabari Smith Jr. as a cornerstone for Rockets' small-ball.

1 hour ago Mikey Musumeci fires back at Dillon Danis after opponent mocking.
1 hour ago US grants Serbia sanctions waiver for NIS oil firm until March 20, 2026.
Popular

Jesse Jackson is an American civil rights activist politician and...

Barack Obama the th U S President - was the...

Bernie Sanders is a prominent American politician currently serving as...

Ken Paxton is an American politician and lawyer serving as...

Michael Joseph Jackson the King of Pop was a highly...
WWE Raw a professional wrestling television program by WWE airs...