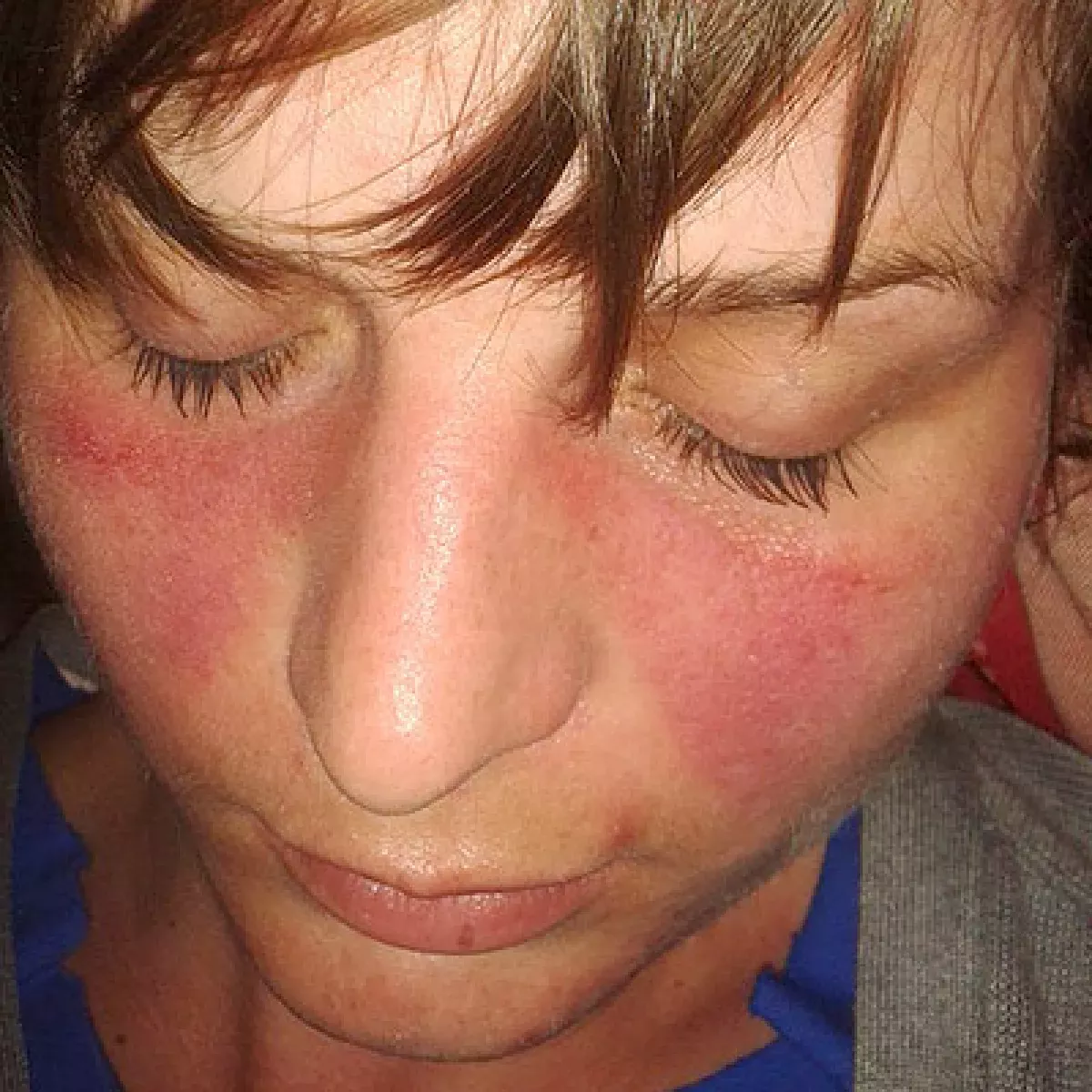
Lupus, or systemic lupus erythematosus (SLE), is an autoimmune disease where the immune system attacks healthy tissue, causing varied symptoms ranging from mild to severe. Common symptoms include joint pain and swelling, fever, chest pain, hair loss, mouth ulcers, swollen lymph nodes, fatigue, and a characteristic facial rash. The disease is characterized by flares (periods of active symptoms) and remissions (periods with few symptoms). A more severe form, childhood-onset SLE, affects individuals up to 18 years old.
1903: SLE afflictions documented by Osler
In 1903, further study of the disease led to a third paper documenting afflictions such as arthritis, pneumonia, the inability to form coherent ideas, delirium, and central nervous system damage as all affecting patients diagnosed with SLE.
1920: Beginning of the modern period in SLE research
The modern period, which began in 1920, saw major developments in research into the cause and treatment of discoid and systemic lupus. Research conducted in the 1920s and 1930s led to the first detailed pathologic descriptions of lupus and demonstrated how the disease affected the kidney, heart, and lung tissue.
1948: Discovery of the LE cell
In 1948, a team of researchers at the Mayo Clinic made a breakthrough with the discovery of the LE cell (lupus erythematosus cell). They found that the white blood cells contained the nucleus of another cell pushing against the white cell's proper nucleus.
1955: FDA approves Hydroxychloroquine for Lupus
In 1955, Hydroxychloroquine was approved by the FDA for lupus treatment and is a first line agent for SLE that is associated with reduced mortality, complications and disease activity.
1955: SLE rate in the United States
In 1955, the rate of SLE in the United States was 1.0.
1968: Drug-induced SLE cases recorded
In 1968, the VigiBase drug safety data repository began recording cases of drug-induced SLE.
1971: American College of Rheumatology (ACR) compiles a list of clinical and immunologic criteria
In 1971, The American College of Rheumatology (ACR) compiled a list of clinical and immunologic criteria that, in any combination, point to SLE.
1974: SLE rate increased in the United States
By 1974, the rate of SLE in the United States had increased to 7.6. The cause of the increase, whether due to better diagnosis or increased disease frequency, remains unknown.
1982: American College of Rheumatology (ACR) establishes criteria for SLE
In 1982, The American College of Rheumatology (ACR) established eleven criteria as a classificatory instrument to operationalise the definition of SLE in clinical trials. They were not intended to be used to diagnose individuals and do not do well in that capacity.
1982: Revision of ACR list of clinical and immunologic criteria
In 1982, The American College of Rheumatology (ACR) list of clinical and immunologic criteria originally compiled in 1971 was revised.
1997: Revision of the American College of Rheumatology (ACR) criteria
In 1997, the American College of Rheumatology (ACR) criteria for SLE, initially established in 1982, were revised as a classificatory instrument for clinical trials.
1998: St. Thomas' Hospital alternative criteria suggested
In 1998, alternative criteria for SLE, such as the St. Thomas' Hospital criteria, were suggested.
2009: Further revision of ACR list of clinical and immunologic criteria
In 2009, The American College of Rheumatology (ACR) list of clinical and immunologic criteria was further revised and improved from the initial compilation in 1971 and revision in 1982.
November 2010: FDA advisory panel recommends approving belimumab (Benlysta)
In November 2010, an FDA advisory panel recommended approving belimumab (Benlysta) as a treatment for the pain and flare-ups common in lupus.
2010: Review of studies correlating race and SLE
In 2010, a review of studies that correlate race and SLE identified several sources of systematic and methodological error, indicating that the connection between race and SLE may be spurious. The review also highlighted the modulating factor of social support against SLE-related damage, suggesting a potential confounding variable in studies correlating race and SLE.
March 2011: FDA approves Belimumab (Benlysta) for lupus
In March 2011, Belimumab (Benlysta) was approved by the FDA as a treatment for lupus.
March 2011: FDA approves Belimumab
In March 2011, Belimumab was approved by the FDA.
2017: Drug-induced SLE cases recorded
By 2017, the VigiBase drug safety data repository had diagnosed 12,166 cases of drug-induced SLE. These cases were linked to 118 different agents, mainly antiarrhythmic, antihypertensive, antimicrobial agents, or those inhibiting interferon or tumor necrosis factor.
2019: Genetically engineered immune cells being studied in animal models
As of 2019, genetically engineered immune cells are also being studied in animal models of the disease.
September 2022: CAR T-cell therapy shows promising results
In September 2022, researchers at the University of Erlangen-Nuremberg published promising results using genetically altered immune cells to treat severely ill patients, where CAR T cells modified to attack their B cells, eliminating the aberrant ones, drove the disease into remission in all five patients.
2024: Classification of inborn errors of immunity genes
As of February 2024, five genes (DNASE1L3, TREX1, IFIH1, Tartrate-resistant acid phosphatase and PRKCD) were classified as inborn errors of immunity genes related to childhood-onset SLE (cSLE). Mutations in approximately 40 genes have been linked to cSLE or cSLE-like diseases.
Mentioned in this timeline
Hydroxychloroquine primarily known by its brand name Plaquenil is a...

A car also known as an automobile is a wheeled...

September is the ninth month of the year in the...
Trending
45 minutes ago Diego Garcia: Britain's Chagos deal faces opposition amid military base concerns and global implications.

45 minutes ago Charles Leclerc and Alexandra Saint Mleux Spark Marriage Rumors in Monaco

2 hours ago Fetterman Discussed Amid Trump's Iran Actions and Lawmaker Support Debate.
2 hours ago Citigroup integrates Bitcoin into $30T asset management, boosting institutional crypto adoption.

2 hours ago Greg Abel's first letter signals Berkshire Hathaway's future post-Buffett, watched by Wall Street.
2 hours ago Ali Larter Celebrates Her 50th Birthday: A Look Back at Her Career
Popular

Jesse Jackson is an American civil rights activist politician and...

Hillary Diane Rodham Clinton is a prominent American politician lawyer...

XXXTentacion born Jahseh Dwayne Ricardo Onfroy was a controversial yet...

Michael Joseph Jackson the King of Pop was a highly...

Barack Obama the th U S President - was the...

Susan Rice is an American diplomat and public official prominent...