Norovirus is a highly contagious virus and the leading cause of gastroenteritis, often called the "winter vomiting disease." Common symptoms include non-bloody diarrhea, vomiting, stomach pain, and sometimes fever or headache, appearing 12-48 hours post-exposure. Recovery usually takes 1-3 days. While complications are rare, dehydration is a concern, particularly for vulnerable populations like the young, elderly, and those with pre-existing health issues. Good hygiene practices are crucial in preventing the spread of Norovirus.
November 1968: Norwalk agent outbreak
In November 1968, an outbreak of acute gastroenteritis occurred among children at Bronson Elementary School in Norwalk, Ohio, leading to the virus being originally named the "Norwalk agent".
1968: Norwalk outbreak
In 1968, an outbreak occurred in Norwalk, Ohio, US, leading to the virus being named after the city.
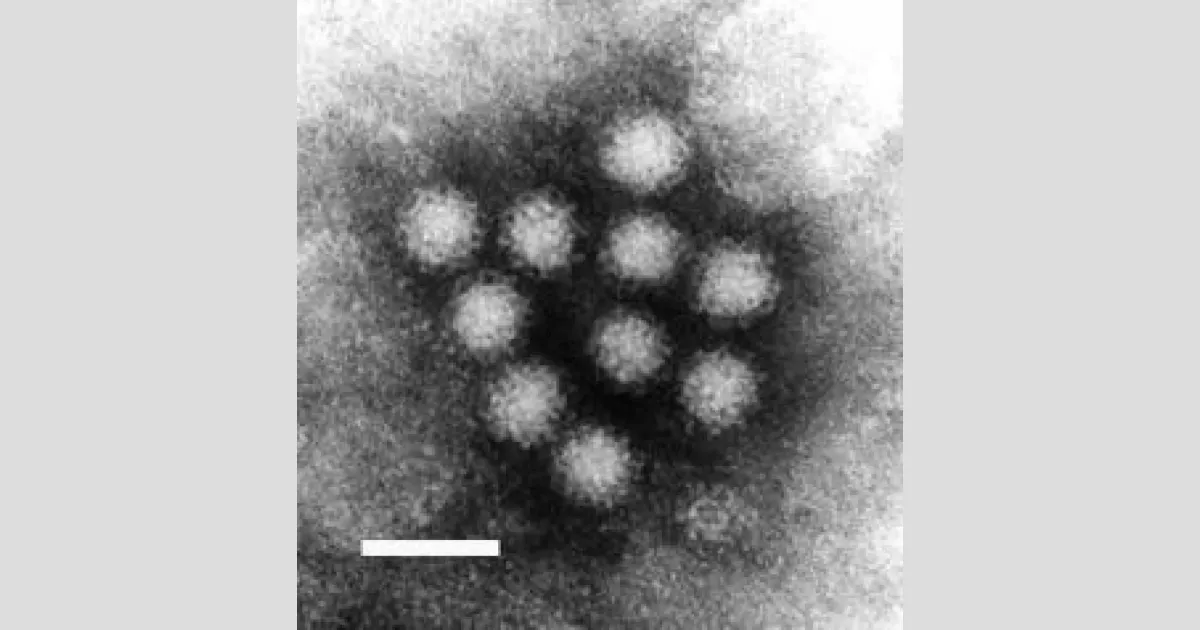
1972: Identification of Norwalk virus
In 1972, electron microscopy on stored human stool samples identified a virus, which was given the name "Norwalk virus".
December 1998: Restaurant Vomiting Incident
In December 1998, an incident occurred in a restaurant where a person vomiting spread the infection across the restaurant, resulting in 52 people reporting symptoms.
2002: Farmington Hills virus outbreak
In 2002, the Farmington Hills virus was associated with norovirus outbreaks in Europe and the United States.
2002: Norovirus name approved
In 2002, the name "norovirus" (Norovirus for the genus) was approved by the International Committee on Taxonomy of Viruses (ICTV).
2004: Farmington Hills virus outbreak recurrence
In 2004, the Farmington Hills virus was again associated with norovirus outbreaks in Europe and the United States.
2006: Norovirus survival on surfaces
A 2006 study found the norovirus remained on surfaces used for food preparation seven days after contamination, highlighting the virus's ability to survive outside a human host for extended periods.
2006: Global increase in NoV infection
In 2006, there was a large increase in Norovirus (NoV) infection around the globe. Studies also showed a link between the expression of human histo-blood group antigens (HBGAs) and susceptibility to norovirus infection, suggesting the capsid of noroviruses may have evolved from selective pressure of human HBGAs.
2007: LigoCyte begins norovirus vaccine trials
In 2007, LigoCyte announced it was working on a norovirus vaccine and had started phase 1 trials.
2011: CDC guideline for norovirus outbreak control
In 2011, the CDC published a clinical practice guideline addressing strategies for the prevention and control of norovirus gastroenteritis outbreaks in healthcare settings, presenting 51 specific evidence-based recommendations organized into 12 categories.
2011: ICTV Press Release Encouraging Use of Norwalk Virus Name
In 2011, the International Committee on Taxonomy of Viruses (ICTV) issued a press release and newsletter encouraging the media, national health authorities, and the scientific community to use the virus name Norwalk virus, rather than the genus name Norovirus, due to concerns about negative associations for people with the family name "Noro" in Japan.
2012: Cruise ship outbreaks
In 2012, there was a previous high in the number of Norovirus outbreaks on cruise ships.
2015: Norovirus Outbreaks on Cruise Ships
In 2015, there were 12 outbreaks of gastrointestinal illness, mostly caused by norovirus, recorded on cruise ships with both a US and foreign itinerary by the CDC's Vessel Sanitation Program.
May 2016: Norovirus Outbreaks on Cruise Ships
From January 1 to May 9 2016, there were 10 outbreaks of gastrointestinal illness, mostly caused by norovirus, recorded on cruise ships with both a US and foreign itinerary by the CDC's Vessel Sanitation Program.
August 2017: Quebec Norovirus Outbreak Linked to Chinese Raspberries
In August 2017, an outbreak of norovirus in Quebec, Canada, sickened over 700 people and was traced to frozen raspberries imported from Harbin Gaotai Food Co Ltd, a Chinese supplier. Canadian authorities issued a recall on raspberry products from Harbin Gaotai.
2019: Bivalent vaccine completes phase 1 trials
As of 2019, a bivalent (NoV GI.1/GII.4) intramuscular vaccine had completed phase 1 trials.
2020: Phase 2b vaccine trials finished
In 2020, the phase 2b trials for the bivalent (NoV GI.1/GII.4) intramuscular vaccine were finished.
2023: Surge in Norovirus Cases on Cruise Ships
According to the CDC, in 2023, there was a surge in norovirus cases on thirteen cruise ships, marking the highest number of outbreaks since 2012.
2023: Estimated Annual Number of Norovirus Cases in the United States
In the United States, the estimated annual number of norovirus cases in 2023 was 21 million, representing a rate of 6,270 cases per 100,000 individuals.
Trending

7 months ago Timberwolves-Thunder Game 4: Gilgeous-Alexander's Impact and How to Watch the Decisive Match
3 months ago Iran faces snapback sanctions from Europe amid nuclear program deadlock and economic fears.
10 days ago Quinnen Williams' Impact on Cowboys' Defense and Resurgence; More Than Just One Player

1 month ago Jerry Jones claims unlocking $100B gas bounty, invests $1B, Prescott understands fan frustration.
Trevon Diggs is a professional American football cornerback currently playing for the Dallas Cowboys in the NFL He was drafted...
Aaron Jones is an American professional football running back currently playing for the Minnesota Vikings Drafted by the Green Bay...
Popular

Candace Owens is an American conservative political commentator and author...

Ilhan Omar is an American politician currently serving as the...

XXXTentacion born Jahseh Dwayne Ricardo Onfroy was a controversial yet...

Tom Cotton is an American politician and Army veteran currently...

Oprah Winfrey an American talk show host television producer actress...
Matt and Ross Duffer known as the Duffer Brothers are...