Regeneron Pharmaceuticals is an American biotechnology company established in 1988 and based in Westchester County, New York. Initially focused on neurotrophic factors and regenerative medicine, the company broadened its research to include cytokine and tyrosine kinase receptors. This expansion led to the development of its first product, a VEGF-trap. The company has grown to become a significant player in the biotechnology industry, developing and commercializing therapies for various diseases.
Mentioned in this timeline

Donald John Trump is an American politician media personality and...

Operation Warp Speed was a US government public-private partnership launched...
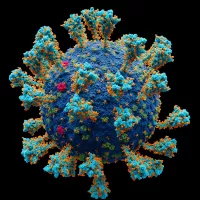
Coronaviruses are a family of RNA viruses affecting mammals and...

Stocks represent fractional ownership of a corporation granting shareholders a...
Trending
9 months ago American Idol Season 23 Finale: Top 3 Compete, John Foster's Journey and Voting Guide

8 months ago Pascal Siakam Shines in NBA Finals, Inspiring Africa and Sharing Lessons.
9 months ago Dengue outbreak in Cook Islands prompts agency response and community clean-ups.

6 months ago Kelly Clarkson's New Bob Haircut and Return to The Voice Season 29!

9 months ago Gavin Newsom faces challenges in final term amid budget concerns and 2028 ambitions.

Gus Bradley is an American football coach currently serving as the defensive coordinator for the Indianapolis Colts He gained recognition...
Popular

Thomas Douglas Homan is an American law enforcement officer who...

William Franklin Graham III commonly known as Franklin Graham is...

Melania Trump a Slovenian-American former model has served as First...

Jupiter is the fifth and largest planet from the Sun...

XXXTentacion born Jahseh Dwayne Ricardo Onfroy was a controversial yet...

Instagram is a photo and video-sharing social networking service owned...
