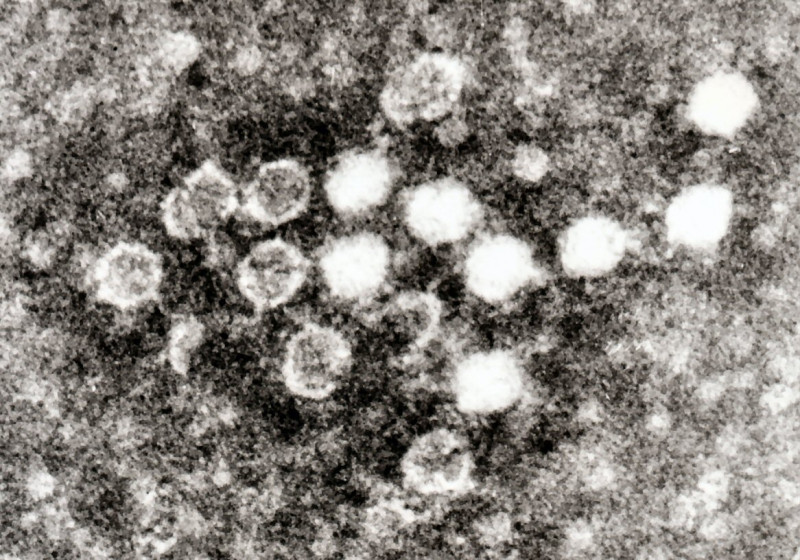
Human parvovirus B19, or B19V, is a tiny DNA virus that commonly affects children, causing fifth disease (erythema infectiosum or "slapped face syndrome"). This childhood rash got its name from being the fifth on a list of historical classifications of common skin rashes in children. While primarily known to affect the pediatric population, B19V can also impact adults.
1975: Discovery of Parvovirus B19
In 1975, Australian virologist Yvonne Cossart discovered the Parvovirus B19, initially identified as a result of serum sample analysis where it was labeled as number 19 in panel B.
1996: Parvovirus B19 Linked to Papular Purpuric Gloves and Socks Syndrome
In 1996, medical researchers identified a connection between Parvovirus B19 and Papular Purpuric Gloves and Socks Syndrome. The virus' presence in skin biopsy samples from individuals with this condition, characterized by itching, swelling, and redness on the hands and feet, provided evidence for this link, later supported by further research.
1998: Parvovirus B19 Epidemic
The year 1998 marked a significant epidemic year for Parvovirus B19, part of its cyclical pattern of increased cases every three to four years. This outbreak particularly affected nurseries and schools.
2011: Clinical Trial for Parvovirus B19 Vaccine Halted
In 2011, Bernstein et al. conducted a clinical trial (Phase I) to investigate a potential vaccine for Parvovirus B19 using virus-like particles (VP2/VP1 VLPs) produced in insect cells. However, the trial was stopped due to unexpected adverse reactions in some participants. Researchers speculated that these reactions might be linked to PLA2 activity in VP1u, either by releasing arachidonic acid and inflammatory mediator precursors or due to the use of insect cells. Despite the setback, reducing or eliminating PLA2 activity through site-directed mutagenesis was proposed as a potential solution for future vaccine development.
2020: No Approved Human Vaccine for Parvovirus B19
As of 2020, there were no approved vaccines for human use against Parvovirus B19.
Mentioned in this timeline

A vaccine is a biological preparation designed to provide active...
Trending
2 months ago Keon Coleman benched for Bills-Bucs game after missing meeting: McDermott explains

2 months ago Meghan Trainor Addresses Weight Loss Criticism Amidst Changing Beauty Standards Debate.
7 months ago Florian Wirtz Reportedly Transfers from Leverkusen to Liverpool for 140 Million Euros

2 months ago James Carville urges Democrats to shift from woke to economic rage.

9 months ago Drew Lock returns to the Seattle Seahawks: Agreement reached for QB comeback.
3 months ago Mel Brooks faces criticism for Hitler joke and UWEC stages his musical.
Popular

XXXTentacion born Jahseh Dwayne Ricardo Onfroy was a controversial yet...

William Franklin Graham III commonly known as Franklin Graham is...
Matt and Ross Duffer known as the Duffer Brothers are...
WWE Raw a professional wrestling television program by WWE airs...
Curt Cignetti is an American college football coach currently the...

Thomas Douglas Homan is an American law enforcement officer who...