Heat, in thermodynamics, is defined as energy transferred between a system and its surroundings through microscopic mechanisms like conduction, radiation, and friction. These involve the motion of sub-atomic, atomic, or molecular particles, contrasting with macroscopic energy transfers like thermodynamic work or matter transfer. For a closed system, the heat involved in a thermodynamic process equals the change in internal energy minus any work done. This relationship is a formulation of the first law of thermodynamics.
Mentioned in this timeline
The letter S in both uppercase and lowercase forms is...
Trending
42 minutes ago Greenland's Ice Churning: Scientists Discover Molten Rock-like Activity Underneath the Ice Sheet.
2 hours ago Sadie Sink and Noah Jupe Star in West End's Romeo & Juliet

2 hours ago A24's 'Marty Supreme' Breaks Records; Costume Design & Cinematography Spotlighted.

3 hours ago Vince McMahon Involved in Horrifying Car Crash: Footage Released
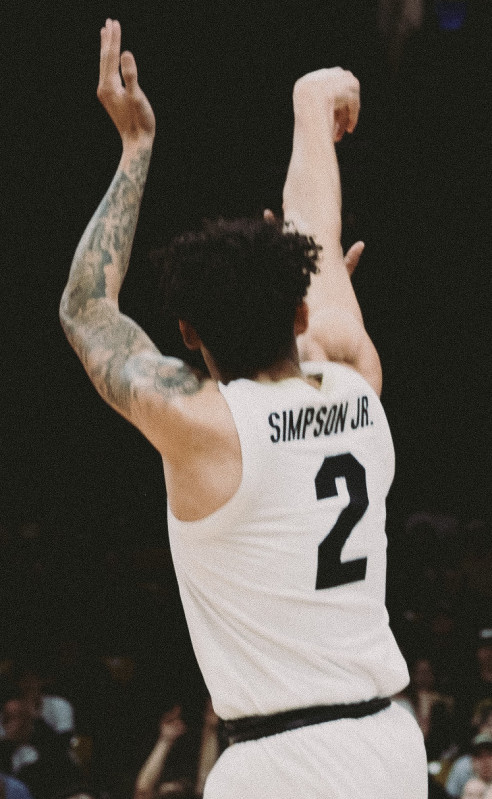
4 hours ago KJ Simpson signs two-way contract with Denver Nuggets after Hornets trade casualty.

3 days ago Deandre Ayton Detained in Bahamas Airport for Marijuana Possession, Later Released.
Popular

Jesse Jackson is an American civil rights activist politician and...
Randall Adam Fine is an American politician a Republican who...

Barack Obama the th U S President - was the...

Ken Paxton is an American politician and lawyer serving as...

Bernie Sanders is a prominent American politician currently serving as...
WWE Raw a professional wrestling television program by WWE airs...
