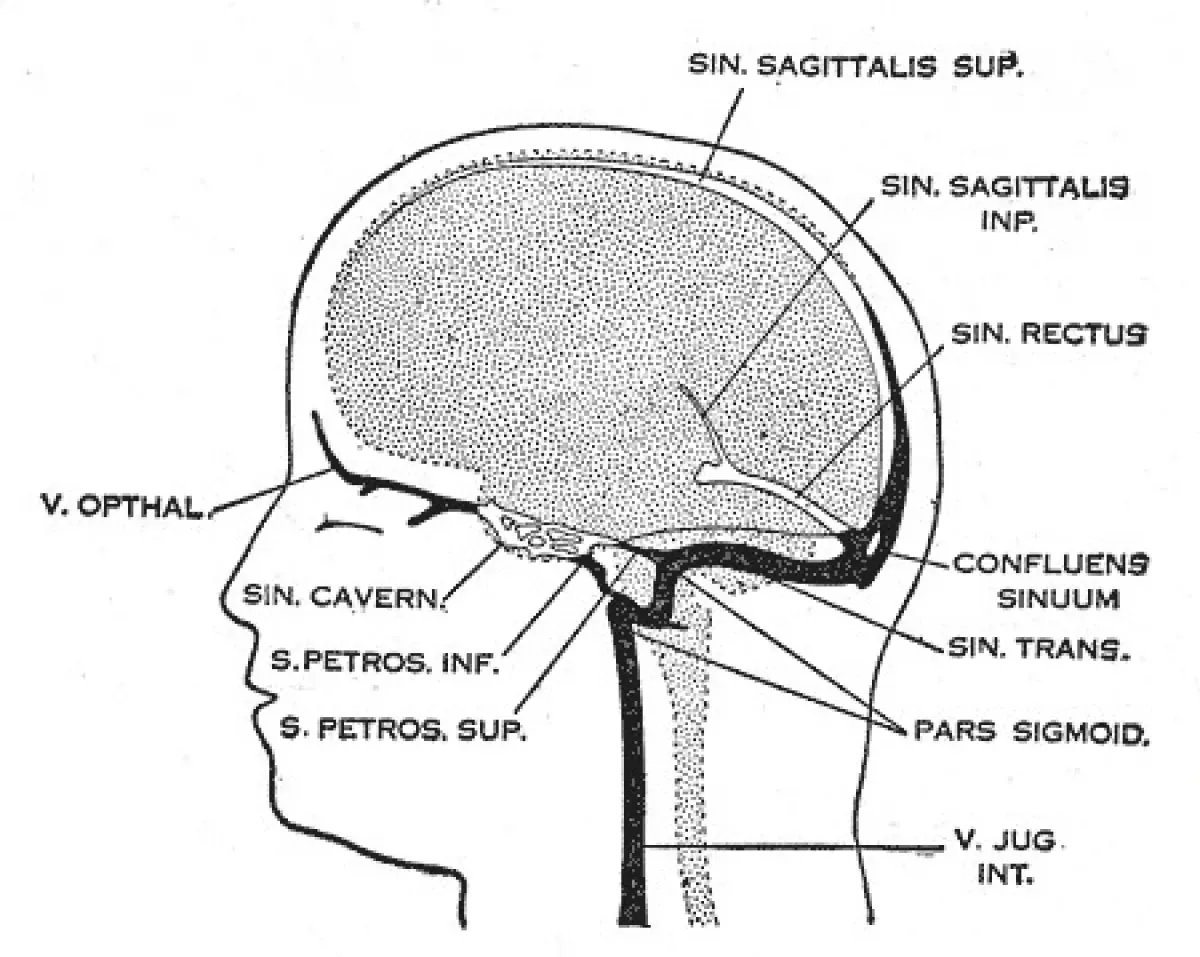
Cerebral venous sinus thrombosis (CVST), also known as cerebral venous and sinus thrombosis or cerebral venous thrombosis (CVT), is a condition characterized by the formation of a blood clot in the dural venous sinuses, cerebral veins, or both. Common symptoms include intense headaches, visual disturbances, stroke-like symptoms such as unilateral weakness, and seizures, which affect approximately 40% of patients.
1942: Stansfield Pioneers Heparin Use in CVST
In 1942, British gynecologist Stansfield introduced the use of heparin, a recently developed anticoagulant, for treating CVST.
1951: Venography Improves CVST Diagnosis
The introduction of venography in 1951 significantly advanced the diagnosis of cerebral venous sinus thrombosis in living patients. This technique also helped differentiate CVST from idiopathic intracranial hypertension, a condition with similar symptoms.
1973: Autopsy Findings and CVST Prevalence
A 1973 report highlighted that CVST was found in 9% of autopsies. Many of these cases involved elderly individuals who had experienced neurological symptoms before death, often accompanied by heart failure.
1995: CVST Incidence Report from Saudi Arabia
In 1995, a report from Saudi Arabia revealed a significantly higher incidence of CVST at 7 cases per 100,000 individuals. This elevated rate was attributed to the prevalence of Behçet's disease in the Middle East, a condition known to increase CVST risk.
2001: Canadian Study on CVST in Children
A 2001 Canadian study provided insights into CVST in children, reporting an incidence of 6.7 cases per million annually. Notably, 43% of cases occurred in newborns (under one month old), and another 10% within the first year of life. Among newborns, 84% were already ill, primarily due to childbirth complications and dehydration.
2004: First Large-Scale Study on CVST Natural History
In 2004, the first adequately sized study examining the natural progression and long-term outcomes of CVST was published. After 16 months, 57.1% of participants had fully recovered, while 29.5% experienced minor symptoms, 2.9% had moderate symptoms, and 2.2% had severe symptoms or impairments. Sadly, 8.3% of the participants died. Factors associated with severe impairment or death included age over 37, male gender, coma, mental status disorders, intracerebral hemorrhage, deep cerebral venous system thrombosis, central nervous system infection, and cancer.
2006: Systematic Review of CVST Studies
A 2006 systematic review of nineteen studies provided further insights into CVST outcomes. The review found that mortality rates were around 5.6% during hospitalization and 9.4% overall. On a positive note, 88% of survivors achieved total or near-total recovery. Within several months, two-thirds of cases showed clot resolution (recanalization), and the recurrence rate remained low at 2.8%.
2006: European Federation of Neurological Societies guideline on Thrombolysis
In 2006, The European Federation of Neurological Societies released a guideline recommending that thrombolysis, a treatment to remove blood clots, only be used in deteriorating CVST patients when other causes have been ruled out. The best drug and administration method remained unclear, and bleeding was a significant concern. Notably, American guidelines did not recommend thrombolysis at this time, citing the need for more research.
December 2012: Hillary Clinton's CVST Diagnosis
In December 2012, U.S. Secretary of State Hillary Clinton was hospitalized for anticoagulation treatment of venous thrombosis in the right transverse sinus, located at the base of her brain. The thrombotic episode was discovered during an MRI scan conducted as a follow-up to a cerebral concussion she sustained 2.5 weeks prior after a fall related to gastroenteritis.
March 2021: European Medicines Agency investigates CVST Cases Potentially Linked to AstraZeneca Vaccine
In March 2021, the European Medicines Agency (EMA) announced that, among roughly 20 million people who received the Oxford–AstraZeneca COVID-19 vaccine, they had identified seven cases of disseminated intravascular coagulation and eighteen cases of cerebral venous sinus thrombosis. While overall blood clotting rates remained normal, the EMA initiated further investigation into a potential causal link with the vaccine, emphasizing that the benefits of vaccination still outweighed the risks.
April 2021: CDC Pauses Janssen COVID-19 Vaccine Due to CVST Cases
On April 13, 2021, the Centers for Disease Control and Prevention (CDC) in the United States temporarily paused the use of the Janssen COVID-19 vaccine due to six reported cases of CVST occurring 6 to 13 days after vaccination. Following a safety review, the recommended pause was lifted on April 23, 2021.
Mentioned in this timeline

Hillary Diane Rodham Clinton is an American politician lawyer and...
Saudi Arabia officially the Kingdom of Saudi Arabia KSA is...
Hypertension commonly known as high blood pressure is a chronic...
COVID- vaccines are designed to provide immunity against SARS-CoV- the...
The heart a muscular organ in humans and animals pumps...

Oxford is a city in Oxfordshire England It serves as...
Trending

Tim Walz is an American politician educator and Army National Guard veteran currently serving as the st Governor of Minnesota...
2 months ago Blinkova and Salkova to face off at the 2025 Jiangxi Open.

2 months ago Alcaraz Rallies to Victory Against Fritz, Closing in on No. 1 Ranking

3 months ago Emma Navarro vs. Shuai Zhang: WTA Wuhan Open 2025 Match Prediction and Preview

2 months ago Deandre Ayton's Impact: Lakers' Undefeated Streak and His Drive After OKC Loss.

1 month ago Deshaun Watson Returns to Browns Practice After Achilles Injury: 21-Day Window Opens
Popular

Stranger Things created by the Duffer Brothers is a popular...

XXXTentacion born Jahseh Dwayne Ricardo Onfroy was a controversial yet...

Kelsey Grammer is an accomplished American actor producer and singer...

Candace Owens is an American conservative political commentator and author...

Bernie Sanders is a prominent American politician currently serving as...

Melania Trump a Slovenian-American former model has served as First...