mRNA vaccines utilize messenger RNA to trigger an immune response. The vaccine introduces antigen-encoding mRNA into cells, instructing them to produce a foreign protein, mimicking a pathogen or cancer cell. This protein stimulates an adaptive immune response, enabling the body to recognize and eliminate the corresponding threat. Lipid nanoparticles encapsulate and protect the mRNA, facilitating its entry into cells. This technology offers a rapid and adaptable approach to vaccine development compared to traditional methods.
1989: First Successful Transfection of mRNA into a Cell
In 1989, the first successful transfection of designed mRNA packaged within a liposomal nanoparticle into a cell was published, marking an important step in mRNA technology.
1993: mRNA Stimulates T Cells in Mice
In 1993, it was shown that liposome-encapsulated mRNA encoding a viral antigen stimulated T cells in mice, demonstrating the potential of mRNA to elicit an immune response.
2001: First Human Clinical Trial of mRNA Cancer Vaccine
In 2001, the first human clinical trial using ex vivo dendritic cells transfected with mRNA encoding tumor antigens (therapeutic cancer mRNA vaccine) was started, marking an early step in the development of mRNA-based cancer therapies.
2008: BioNTech Founded
In 2008, BioNTech was founded to develop mRNA biotechnologies, playing a key role in the advancement of mRNA technology.
2008: Clinical Trial Results of mRNA Vaccine Against Cancer Cells
In 2008, clinical trial results of an mRNA vaccine directly injected into the body against cancer cells were reported, contributing to the growing body of research on mRNA vaccines for cancer treatment.
2010: Moderna Founded
In 2010, Moderna was founded with the goal of developing mRNA biotechnologies, marking a significant step in the growth of the mRNA field.
2013: First Human Clinical Trials of mRNA Vaccine Against Infectious Agent
In 2013, the first human clinical trials using an mRNA vaccine against an infectious agent (rabies) began, marking a significant milestone in the development of mRNA vaccines for infectious diseases.
2018: FDA Approves First Lipid Nanoparticle Drug Delivery System
In 2018, the FDA approved the use of lipid nanoparticles as a drug delivery system with the approval of Onpattro, the first siRNA drug. This was a critical breakthrough for producing viable mRNA vaccines.
November 2020: Report on Thermostability Differences of mRNA Vaccines
In November 2020, Nature reported on the thermostability differences between the Pfizer–BioNTech's BNT162b2 and Moderna's mRNA-1273 vaccines, suggesting they might have similar storage requirements despite initial claims.
December 2020: Authorization of mRNA-based COVID-19 vaccines
In December 2020, Pfizer–BioNTech and Moderna received authorization for their mRNA-based COVID-19 vaccines, marking a significant milestone in the use of mRNA technology to combat the COVID-19 pandemic.
2020: Debate on mRNA Vaccine Authorization
In 2020, the COVID-19 pandemic led to debate about the type of initial authorization mRNA vaccines should receive, including emergency use authorization or expanded access authorization, due to the need for faster production and potential risks.
2020: Challenges in Scaling Up Lipid Production for mRNA Vaccines
In 2020, the companies manufacturing lipids for lipid nanoparticles faced the challenge of scaling up production to meet the demand for mRNA vaccines, highlighting a bottleneck in the large-scale use of this delivery method.
2020: Approval of mRNA-based COVID-19 vaccines
In December 2020, BioNTech and Moderna obtained approval for their mRNA-based COVID-19 vaccines. The UK Medicines and Healthcare products Regulatory Agency (MHRA) approved the Pfizer–BioNTech's BNT162b2 COVID-19 vaccine first, followed by the US FDA giving emergency use authorization for the Pfizer–BioNTech COVID-19 vaccine and subsequently the Moderna COVID-19 vaccine. This marked a major turning point for mRNA vaccine technology.
June 2021: FDA Adds Warning About Myocarditis and Pericarditis Risk
In June 2021, the US FDA added a warning about the possibility of increased risk of myocarditis and pericarditis for some people after receiving mRNA COVID-19 vaccines, highlighting potential side effects.
2021: Phase 1 Trials of saRNA COVID-19 Vaccines Started
In 2021, GSK and Gritstone bio initiated Phase 1 trials of saRNA COVID-19 vaccines, showcasing the ongoing efforts to develop more advanced vaccine technologies.
June 2022: Authorization of Gemcovac in India
In June 2022, Gemcovac became the first saRNA COVID vaccine authorized, marking a milestone in vaccine development in India.
November 2023: Authorization of ARCT-154 in Japan
In November 2023, a version of ARCT-154, developed by Arcturus Therapeutics and manufactured by Meiji Seika Pharma, was authorized in Japan, signifying further advancements in saRNA vaccine technology.
2023: Nobel Prize Awarded for mRNA Vaccine Discoveries
In 2023, Katalin Karikó and Drew Weissman were awarded the Nobel Prize in Physiology or Medicine for their discoveries concerning modified nucleosides. Their work enabled the development of effective mRNA vaccines against COVID-19, marking a significant achievement in the field.
2023: Interim Results of Gritstone bio's saRNA COVID-19 Vaccine Trial
In 2023, interim results of Gritstone bio's Phase 1 trial of an saRNA COVID-19 vaccine, used as a booster vaccine, were published, contributing to the understanding of saRNA vaccine potential.
Mentioned in this timeline
Pfizer Inc is a multinational pharmaceutical and biotechnology corporation headquartered...
India officially the Republic of India is located in South...
Japan is an East Asian island country in the Pacific...
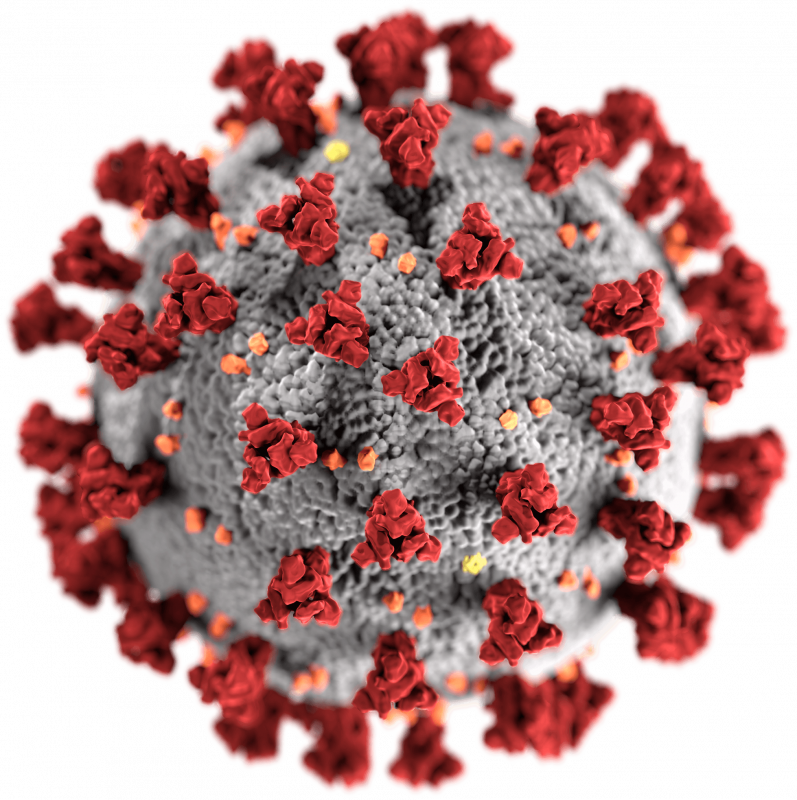
COVID- vaccines are designed to provide immunity against SARS-CoV- the...
Cancer is a collective term for diseases characterized by abnormal...

Drew Weissman is an American physician and immunologist renowned for...
Trending

6 months ago Devin Booker and Molly Murphy spark dating rumors at Stagecoach 2025.

44 minutes ago Tate McRae secures first Grammy nomination with 'Just Keep Watching,' album misses nod.

1 month ago Addison Rae on the Tonight Show: Interview and performance highlight rising star.

5 months ago Kid Rock Opens Nashville Seafood Restaurant, Receives Congratulations from Trump Who Calls Him 'badass'.

45 minutes ago Nathaniel Rateliff's Album Impact and Rock & Roll Hall of Fame Induction Details.

6 months ago Avril Lavigne and Simple Plan reunite for 'Young & Dumb,' recalling 2003 meeting.
Popular

Nancy Pelosi is a prominent American politician notably serving as...

Zohran Kwame Mamdani is an American politician currently serving as...

William Franklin Graham III commonly known as Franklin Graham is...

Chuck Schumer is the senior United States Senator from New...
Nicholas J Fuentes is a far-right political commentator and activist...

Gavin Newsom is an American politician and businessman currently serving...