Eli Lilly and Company, founded in 1876 by pharmaceutical chemist and Civil War veteran Eli Lilly, is an American multinational pharmaceutical corporation headquartered in Indianapolis, Indiana. Operating in 18 countries, its products are marketed in around 125 nations. The company is named after its founder, Eli Lilly.
1905: Company expansion and increased sales
In 1905, under the supervision of J. K. Lilly, Eli Lilly oversaw a significant expansion of the company, leading to annual sales reaching $1 million.
1909: Introduction of blueprinting method for manufacturing tickets
In 1909, Eli Lilly introduced a method for blueprinting manufacturing tickets, which created multiple copies of a drug formula and helped eliminate manufacturing and transcription errors.
1911: New Science Building opening
In 1911, as part of its expanded manufacturing facilities at the McCarty Street plant, Eli Lilly opened a new Science Building (Building 14), improving its production capacity.
1913: Capsule plant and Lilly Biological Laboratories construction
In 1913, Eli Lilly opened a new capsule plant (Building 15) at the McCarty Street plant and began construction of Lilly Biological Laboratories, a research and manufacturing plant near Greenfield, Indiana.
1917: Lilly as "the largest capsule factory in the world"
In 1917, Scientific American described Lilly as "the largest capsule factory in the world" and reported that the company could produce 2.5 million capsules a day.
1919: Hiring of George Henry Alexander Clowes
In 1919, Josiah K. Lilly hired biochemist George Henry Alexander Clowes as director of biochemical research for Eli Lilly.
December 1921: Clowes proposes collaboration with University of Toronto scientists
In December 1921, George Clowes proposed a collaboration between Eli Lilly and University of Toronto scientists (John Macleod, Frederick Banting, and Charles Best) to develop insulin for treating diabetes.
May 1922: Meeting to negotiate insulin production agreement
In May 1922, George Clowes and Eli Lilly met with the University of Toronto scientists to negotiate an agreement for Eli Lilly to mass-produce insulin, which accelerated its large-scale production.
1923: Nobel Prize awarded for insulin research
In 1923, Banting and Macleod were awarded the Nobel Prize for their research on insulin, which they shared with co-discoverers Charles Best and James Collip. Eli Lilly subsequently enjoyed an effective monopoly on the sale of insulin in the U.S.
1923: Selling Iletin
In 1923, Eli Lilly began selling Iletin, its tradename for the first commercially available insulin product in the U.S. for the treatment of diabetes.
June 1924: End of Lilly's monopoly on insulin sales
In June 1924, Eli Lilly's effective monopoly on the sale of insulin in the U.S. ended when Frederick Stearns & Co., the first of the new American licensees, entered the market.
1926: Opening of Building 22 with straight-line production
In 1926, Eli Lilly opened Building 22 in Indianapolis, implementing the new concept of straight-line production to improve efficiency and lower production costs.
1926: Company's 50th anniversary and sales
In 1926, on its 50th anniversary, Eli Lilly's sales reached $9 million, and the company was producing over 2,800 products.
1928: Introduction of Liver Extract 343
In 1928, Eli Lilly introduced Liver Extract 343 for the treatment of pernicious anemia in a joint venture with George Minot and William P. Murphy from Harvard University.
1930: Introduction of Liver Extract No. 55
In 1930, Eli Lilly introduced Liver Extract No. 55 in collaboration with George Whipple, a scientist from the University of Rochester.
1930: Merthiolate introduction
In 1930, Eli Lilly introduced Merthiolate, an "antiseptic and germicide" that became a U.S. Army standard issue during World War II.
1930: Launch of Merthiolate
In 1930, Lilly launched merthiolate, a mercury-based antiseptic and germicide formulated at the University of Maryland. Merthiolate, also known as thiomersal, was effective by causing susceptible bacteria to autolyze.
1932: Eli Lilly becomes company president
In 1932, Eli Lilly, eldest grandson of Col. Lilly, was named as the company's president, succeeding his father.
1934: Establishment of first overseas subsidiary
In 1934, Eli Lilly and Company Limited, the company's first overseas subsidiary, was established in London, and a manufacturing plant was opened in Basingstoke.
1934: New Research Laboratory in Indianapolis
In 1934, Eli Lilly built a new research laboratory in Indianapolis. As part of its research and product development process Lilly also conducted clinical studies at Indianapolis City Hospital. Lilly continues to conduct clinical studies to test medications before their introduction to the market.
1934: Opening of new facilities on the McCarty Street complex
In 1934, Eli Lilly opened a replica of Lilly's 1876 laboratory and the new Lilly Research Laboratories, described as "one of the most fully equipped facilities in the world," on the McCarty Street complex.
1934: Nobel Prize in Physiology or Medicine awarded to Minot, Murphy, and Whipple
In 1934, George Minot, William P. Murphy, and George Whipple were awarded the Nobel Prize in Physiology or Medicine for their research, which Lilly collaborated on.
1943: Formation of Eli Lilly International Corp.
In 1943, Eli Lilly International Corp. was formed as a subsidiary to encourage business trade abroad.
1944: Company Reorganization
In 1944, Eli Lilly and Company announced a reorganization after three generations of Lilly family leadership. This reorganization prepared the way for future expansion and the eventual separation of company management from its ownership.
1945: Major expansion effort begins
In 1945, Eli Lilly began a major expansion effort that included two manufacturing operations in Indianapolis.
1947: Introduction of Dolophine
In 1947, Eli Lilly introduced methadone to the United States under the trade name "Dolophine." Lilly acquired the rights to produce the drug due to the seizure of German patents after World War II. The drug is an analgesic used frequently in the treatment of heroin, opium and other opioid and narcotic drug addictions.
1947: Completion of Kentucky Avenue renovation
In mid-1947, after renovation, the Kentucky Avenue location manufactured antibiotics and capsules and housed the company's shipping department.
1948: Lilly employs nearly 7,000 people
By 1948, Eli Lilly employed nearly 7,000 people as a result of the company's expansion efforts.
1948: International expansion during World War II
By 1948, Eli Lilly employees worked in 35 countries, mostly as sales representatives in Latin America, Asia, and Africa, further expanding international operations during World War II.
1948: Eli Lilly retires and Josiah K. Lilly Jr. becomes president
In 1948, Eli Lilly, who served as the company's president since 1932, retired from active management and became chairman of the board, relinquishing the presidency to his brother, Josiah K. Lilly Jr.
1948: Chairman of the board
In 1948, his father remained as chairman of the board.
1949: Partnership with the United States Army Reserve
In 1949, Eli Lilly partnered with the United States Army Reserve to establish a local Strategic Intelligence Research and Analysis (SIRA) Unit, enabling employees to research company data for scientific logistics and Eurasian studies.
1950: Launch of Tippecanoe Laboratories and increased antibiotic production
In 1950, Eli Lilly launched Tippecanoe Laboratories in Lafayette, Indiana, and increased antibiotic production with its patent on erythromycin.
1952: First public shares of stock
In 1952, Eli Lilly offered its first public shares of stock, which were traded on the New York Stock Exchange.
1953: Eugene N. Beesley named company president
In 1953, Eugene N. Beesley was named Eli Lilly's new president, the first non-family member to run the company.
1953: Josiah K. Lilly Jr. becomes chairman of the board
In 1953, Josiah K. Lilly Jr. stepped down as president and became chairman of the board.
1953: Company reorganization and continued global expansion
In 1953, following a company reorganization and transition to non-family management, Eli Lilly continued to expand its global presence.
1954: Formation of Elanco Products Company
In 1954, Eli Lilly formed Elanco Products Company, named after its parent company, for the production of veterinary pharmaceuticals.
1954: Contract with NFIP to produce Salk's polio vaccine
In 1954, the National Foundation for Infantile Paralysis contracted with Eli Lilly and four other pharmaceutical companies to produce Salk's polio vaccine for clinical trials.
1955: Polio vaccine mass production
In 1955, Eli Lilly became the first company to mass-produce the polio vaccine developed by Jonas Salk.
1955: Lilly manufactures 60% of Salk's polio vaccine
In 1955, Eli Lilly manufactured 60 percent of Salk's polio vaccine.
1966: Death of Josiah K. Lilly Jr.
In 1966, Josiah K. Lilly Jr. remained chairman of the board until his death.
1966: Valley of the Dolls Release
In 1966, the novel "Valley of the Dolls" by Jacqueline Susann featured secobarbital as a central part of the plot, highlighting the drug's impact on Hollywood culture. A film was later released based on the novel.
June 1969: Judy Garland's Death by Secobarbital Overdose
In June 1969, actress Judy Garland died from a secobarbital overdose, bringing attention to the recreational use and potential dangers of the drug.
1969: End of Lilly Family as Chairman of the Board
In 1969, Lilly family members ceased to serve as chairman of the board, marking a transition to non-family management within the company.
September 1970: Jimi Hendrix's Death by Secobarbital Overdose
In September 1970, rock guitarist Jimi Hendrix died from a secobarbital overdose, bringing attention to the recreational use and potential dangers of the drug.
1972: Richard Donald Wood Appointed President and CEO
In 1972, Richard Donald Wood became Lilly's president and CEO after the retirement of Burton E. Beck, continuing the shift towards non-family management.
1986: Randall L. Tobias Joins Lilly Board
In 1986, Randall L. Tobias, then a vice chairman of AT&T Corporation, became a member of the Lilly board.
1987: FDA Approval of Prozac
In 1987, the US FDA approved Prozac for use in treating depression, marking the introduction of one of the first therapies in its class to treat clinical depression by blocking the uptake of serotonin within the human brain.
1991: Vaughn Bryson Becomes President and CEO
In 1991, Vaughn Bryson became president and CEO of Eli Lilly, with Richard Wood becoming board chairman. During Bryson's tenure, the company reported its first quarterly loss as a publicly traded company.
June 1993: Randall L. Tobias Named Chairman, President, and CEO
In June 1993, Randall L. Tobias was appointed as chairman, president, and CEO of Eli Lilly, recruited from AT&T Corporation. He replaced Vaughn Bryson as president and CEO, and then also replaced Richard Wood as board chairman.
1996: Release of Zyprexa
In 1996, Zyprexa (Olanzapine) was released for the treatment of schizophrenia and bipolar disorder, eventually becoming the company's best-selling drug through 2010.
1996: FDA Approval of Gemzar
In 1996, the U.S. Food and Drug Administration approved gemcitabine (Gemzar) for the treatment of pancreatic cancer. Gemzar is commonly used in the treatment of pancreatic cancer, usually in coordination with 5-FU chemotherapy and radiation therapy. Gemzar also is routinely used in the treatment of non-small cell lung cancer.
July 1998: Sidney Taurel Named CEO
In July 1998, Sidney Taurel was appointed CEO of Eli Lilly, succeeding Randall Tobias. He had previously served as the company's chief operating officer.
1998: New Clinical Research Laboratories
In 1998, Eli Lilly dedicated new laboratories for clinical research at the Indiana University Medical Center in Indianapolis.
January 1999: Taurel becomes chairman of the board
In January 1999, Sidney Taurel became chairman of the board.
1999: Cessation of Secobarbital Manufacturing
In 1999, Eli Lilly ceased manufacturing Secobarbital (Seconal) due to the onset of new therapies for the treatment of conditions it was indicated for.
November 2002: Protection from Thiomersal Lawsuits
In November 2002, congressional Republicans inserted a provision into a domestic security bill signed into law by President George W. Bush, protecting Eli Lilly from all suits in the federal courts, alleging that the drug, Thiomersal caused autism and other neurological disorders in children.
2002: Generic versions of Prozac appearing
After 2002, generic versions of Prozac started appearing, after patent expiry.
2003: Introduction of Cialis
In 2003, Eli Lilly and Company introduced Cialis (tadalafil), a competitor to Pfizer's Viagra for erectile dysfunction. Cialis maintains an active period of 36 hours. Cialis was developed in a partnership with biotechnology company Icos Corporation.
October 2005: Lechleiter becomes Lilly's president and chief operating officer
In October 2005, John C. Lechleiter had served as Lilly's president and chief operating officer.
December 2006: Acquisition of Icos Corporation
In December 2006, Lilly bought Icos in order to gain full control of Cialis.
March 2008: Sidney Taurel Retires as CEO
In March 2008, Sidney Taurel retired as CEO of Eli Lilly but remained as chairman of the board until December 31, 2008.
April 2008: John C. Lechleiter Elected CEO and President
On April 1, 2008, John C. Lechleiter was elected as Lilly's CEO and president, succeeding Sidney Taurel. Lechleiter had served as Lilly's president and chief operating officer since October 2005.
December 2008: Sidney Taurel Retires as chairman of the board
In December 2008, Sidney Taurel retired as chairman of the board.
2008: Research projects within the Innovative Medicines Initiative
In 2008, Lilly's activities included research projects within the framework of the Innovative Medicines Initiative, a public-private research initiative in Europe that is a joint effort of the EFPIA and the European Commission.
2008: Securing Budget for 40-Month Project
In 2008, a consortium, which included Lilly S.A. in Switzerland, secured an €8 million budget for a 40-month project coordinated by the European Federation of Pharmaceutical Industries and Associations (EFPIA).
2009: Illegal marketing of Zyprexa and penalty
In 2009, Eli Lilly pleaded guilty to illegally marketing Zyprexa and agreed to pay a $1.415 billion penalty, which included a criminal fine of $515 million, the largest ever in a healthcare case at the time.
2010: Patent expiration of Zyprexa
In 2010, the patent for Zyprexa expired after being the company's best selling drug, affecting its market position.
2020: Introduction of Lilly Insulin Value Program
In 2020, Lilly introduced the Lilly Insulin Value Program, enabling individuals with commercial insurance or no insurance to access any Lilly insulin for $35 per month through a savings card.
January 2022: Pause of COVID-19 Antibody Drug Distribution
In January 2022, the distribution of Lilly's COVID-19 antibody drug was paused due to its lack of efficacy against the emerging omicron variant.
February 2022: Emergency Use Authorization for Bebtelovimab
In February 2022, bebtelovimab, a second COVID-19 monoclonal antibody therapy developed with AbCellera, was granted Emergency Use Authorization. The U.S. government committed to a $720 million purchase of up to 600,000 doses.
May 2022: FDA Approval of Mounjaro
In May 2022, the FDA approved Lilly's type 2 diabetes drug Mounjaro (tirzepatide).
August 2022: Response to Indiana Abortion Ban
In August 2022, following the overturning of Roe v. Wade and the passage of a near-total abortion ban in Indiana, Lilly expressed concerns that the move would hinder talent acquisition and force the company to seek "more employment growth" elsewhere, impacting the state's economy.
October 2022: Acquisition of Akouos Inc.
In October 2022, Eli Lilly announced it would acquire Akouos Inc. for $487 million in upfront and $123 million deferred payments.
January 2023: Collaboration and License Agreement with TRexBio
In January 2023, Lilly and TRexBio announced a collaboration and license agreement for three assets to treat immune-mediated diseases, with TRexBio receiving an upfront payment of $55 million as part of the deal.
March 2023: $35 Cap on Monthly Insulin Price
In March 2023, Eli Lilly announced a $35 cap on the price of monthly insulin to be put in place immediately in order to be in line with the Inflation Reduction Act.
July 2023: Acquisition of Versanis
In July 2023, Lilly announced it would acquire Versanis for $1.93 billion.
October 2023: Acquisition of Point Biopharma
In October 2023, Eli Lilly acquired Point Biopharma for $1.4 billion.
November 2023: FDA Approval of Zepbound
In November 2023, the FDA approved tirzepatide for the treatment of obesity under the brand name Zepbound.
2023: Inflation Reduction Act Extends Insulin Cost Cap
In 2023, the Inflation Reduction Act expanded the concept of insulin cost caps across all insulin suppliers, limiting out-of-pocket costs to $35 per monthly prescription for Medicare Parts B and D enrollees.
March 2024: Partnership with Amazon for Home Delivery
In March 2024, Lilly announced a deal with Amazon to offer home delivery of certain medications for diabetes, obesity, and migraines, on behalf of LillyDirect.
October 2024: Lilly's market capitalization
As of October 2024, Eli Lilly and Company became the most valuable drug company in the world, reaching a market capitalization of $842 billion, the highest valuation ever achieved by a drug company.
October 2024: Eli Lilly Becomes Most Valuable Drug Company
As of October 2024, tirzepatide's success as a blockbuster weight-loss drug had transformed Eli Lilly into the most valuable drug company in the world with a $842 billion market capitalization, the highest valuation ever achieved by a drug company to date, followed only by Novo Nordisk.
2024: FDA approval of Kisunla
In 2024, Kisunla (donanemab), a monoclonal antibody used for the treatment of Alzheimer's disease, was approved by the US FDA.
Mentioned in this timeline

Liverpool is a port city and metropolitan borough located in...
The United States of America is a federal republic located...
California is a U S state on the Pacific Coast...

Judy Garland was a celebrated American actress singer and vaudevillian...
Pfizer Inc is a multinational pharmaceutical and biotechnology corporation headquartered...
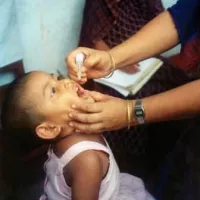
Polio vaccines are used to prevent poliomyelitis There are two...
Trending

7 days ago Karl-Anthony Towns Expresses Desire to Extend Stay with Knicks Amid Contract Stalls

6 days ago Bam Adebayo Unhappy with Anthony Davis Trade Rumors, NBA Volatility Impacting Players

3 days ago Tottenham vs Brentford and Man City vs Sunderland: Premier League Football Action
7 months ago Galatasaray vs Kayserispor: Match Details, Championship Hopes, and Okan Buruk's Strategy

Mason Greenwood is an English professional footballer currently playing as a forward for Marseille in Ligue He rose through the...
4 months ago Eli Lilly's Obesity Pill Shows Promising Weight Loss Results in Phase 3 Trial
Popular
Matt and Ross Duffer known as the Duffer Brothers are...

Candace Owens is an American conservative political commentator and author...

Ilhan Omar is an American politician currently serving as the...

XXXTentacion born Jahseh Dwayne Ricardo Onfroy was a controversial yet...

Tom Cotton is an American politician and Army veteran currently...

Harriet Tubman was a pivotal American abolitionist and social activist...
